Covid: Aifa approva il vaccino bivalente Comirnaty sviluppato contro Omicron 4-5 | Sanità24 - Il Sole 24 Ore

Moderna su LinkedIn: Moderna's Omicron-Containing Bivalent Booster Candidate, mRNA-1273.214… | 13 commenti

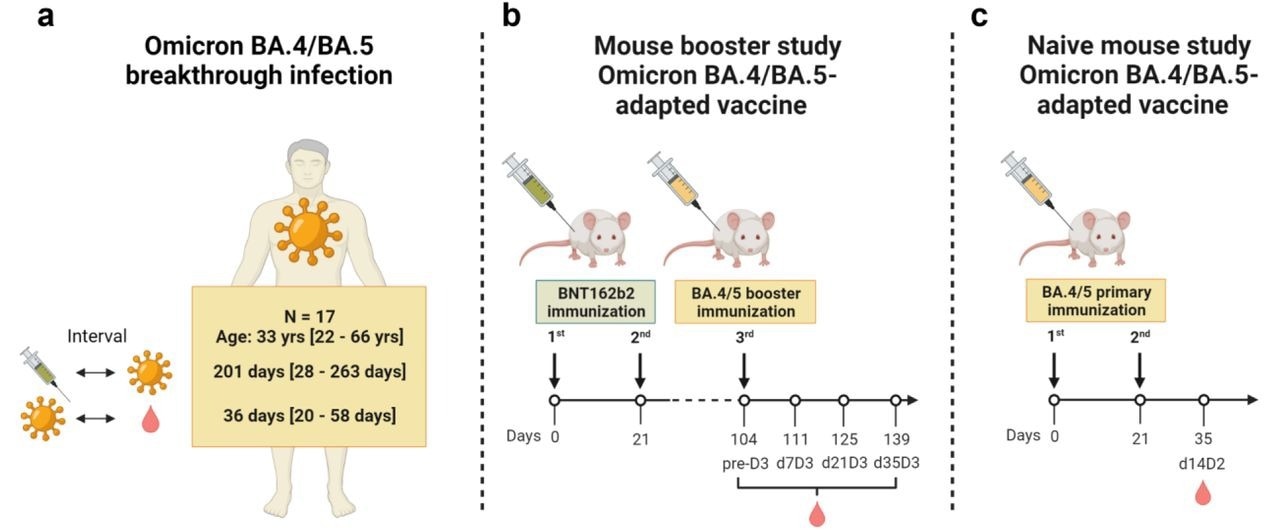
Exposure to BA.4/5 S protein drives neutralization of Omicron BA.1, BA.2, BA.2.12.1, and BA.4/5 in vaccine-experienced humans and mice | Science Immunology

Comparative effectiveness of bivalent BA.4-5 and BA.1 mRNA booster vaccines among adults aged ≥50 years in Nordic countries: nationwide cohort study | The BMJ

Omicron boosters: Do I need another COVID-19 shot, and when? | COVID | Prevention | UT Southwestern Medical Center

Moderna on X: "ANNOUNCEMENT 📢: The first participant has been dosed in a Phase 2/3 trial of mRNA-1273.222, a bivalent #COVID19 #booster vaccine candidate targeting the original and #Omicron BA.4/5 strains of

AIFA autorizza il vaccino bivalente Comirnaty Original per le varianti Omicron BA.4-5 – Centro Regionale FarmacoVigilanza Sardegna
Bivalent BA.4-5 or BA.1 mRNA-booster given as a fourth dose associated with increased protection against COVID-19 hospitalization and death

News - CHMP Recommends Additional Authorisation Modification of Comirnaty (BioNTech/Pfizer) As a Bivalent Vaccine Adapted to Omicron BA.4/BA.5 for Booster Vaccinations - Paul-Ehrlich-Institut
Common Questions About Bivalent COVID-19 Boosters | Johns Hopkins | Bloomberg School of Public Health
BioNTech SE on LinkedIn: Pfizer and BioNTech Report New Data on Omicron BA.4 /BA.5-Adapted Bivalent…

CDC on X: "The updated #COVID19 booster can both help restore protection that has waned since the previous vaccination and provide broader protection against newer BA.4 and BA.5 Omicron subvariants. Learn more:
Updated Covid-19 boosters offer protection, but new studies suggest they don't offer an edge against Omicron | CNN